
Unlike cement, lime mortars and its related products re-absorb carbon dioxide emissions during the production process and continue to recarbonate CO2 over its in-use phase, creating a complete life cycled, closed-loop process, resulting in negative-zero (-2%) carbon emissions.
- Up to 7 billion tons of alkaline materials are produced globally each year as a product or by-product of industrial activity. The aqueous dissolution of these materials creates high pH solutions that dissolves CO2 that store carbon in the form of solid carbonate minerals or dissolved bicarbonate ions.
- Due to the chemically bonded existence of cement, carbonate minerals are trapped within the matrix and do not freely absorb and release CO2 in the environment.
- Carbonate minerals found in calcium carbonate and calcium hydroxide (limestone & lime) freely exchange CO2 with the environment during production and in the product life cycle.
- Cement-based building products absorb only up to 20% of its produced carbon output resulting in partially sustainable end-use materials.
- Although cement contains lime and limestone (calcium carbonate and calcium hydroxide) – its highly solid, non-crystalline nature traps free moving carbonates, reducing its ability to cyclically ‘recarbonate’ CO2. (see Lime Science)
- Lime-based building products that have very low-to-no concentrations of Portland cement perform differently than their high cement-based counterparts.
Cement vs. Lime production
Cement is produced by heating limestone and a source of silicon (clay/shale) in a kiln at 2000° C to produce metastable calcium silicate minerals (clinker, e.g., Ca2SiO4). The clinker is hydrated during construction to produce mortar and concrete. These high-strength products provide solutions for modern architecture and infrastructure, however, they fall short of being environmentally responsible. Cement and its byproducts (i.e. Portland Cement) do not repurpose easily in reconstruction and do not reconstitute soundly in landfill.
Like cement, lime is produced by heating limestone (although, solely) at 900° C in a kiln, but is subsequently used in numerous industries besides cement and clinker. Of the lime produced in the United States and European Union, 30–40% is used by the steel industry as a fluxing agent, 14% is used in other industries (sugar refining, glass, paper, precipitated calcium carbonate), 10–20% is used in construction and 16–24% is used for environmental remediation/treatment (flue gas desulphurisation, water treatment, acid mine drainage). Approximately 20% of lime is used in activities that exploit reactions with CO2 and weathering (e.g., agricultural liming, lime-based building products (bricks/blocks, coatings/finishes). Approximately 14% of the lime is used in activities that do not have an explicit reaction with CO2, but it may be possible to engineer this within the life cycle of the material (soda lime glass, soil stabilization).

Limestone firing in a kiln 
Limestone cooking at 900°c
Lime/Limestone-based building products that do not use Portland cement, recapture CO2 from the atmosphere once off gassed during production and even reduce carbon emissions from overall cement manufacturing.
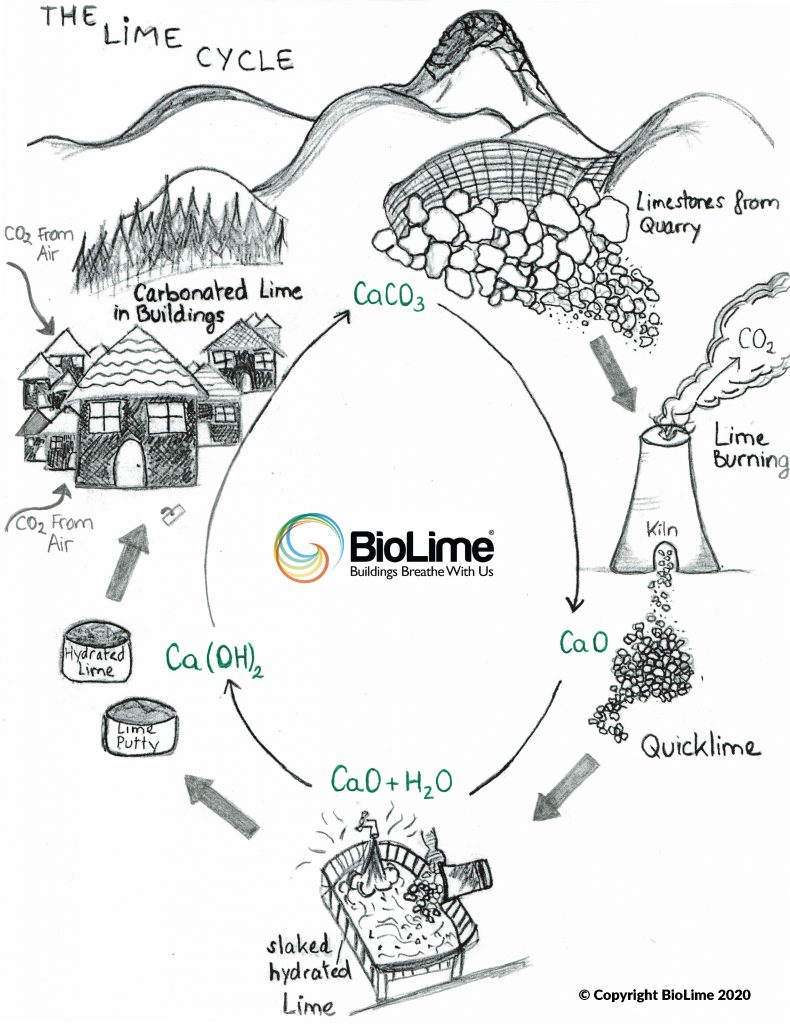
Like cement, lime gives off carbon dioxide during manufacture. Yet, unlike cement, lime mortars and its related products re-absorb carbon dioxide during the production process and continue to recarbonate CO2 over its in-phase use, creating a complete life cycled, closed-loop process.
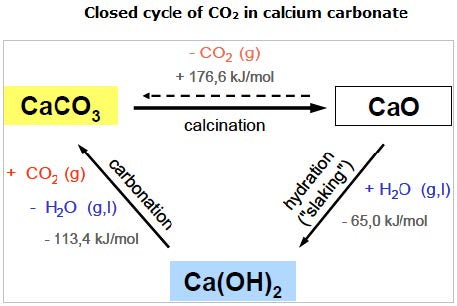
Source: EuLA (European Lime Assoc.) / TU Clausthal, 2008
The Science of Lime
Recarbonation is the reabsorption and release of carbon dioxide (CO2) from the atmosphere during a materials in-use phase. Studies show that recarbonation accounts for an average of 20 per cent of the carbon dioxide emitted during the production phase of concrete, reducing its whole life CO2 impact. As shown below, the spectrographic image of cement is “chemically-locked” and encapsulates the pore structure within the matrix, not allowing free-moving crystal calcium (lime and limestone) to absorb and reabsorb.
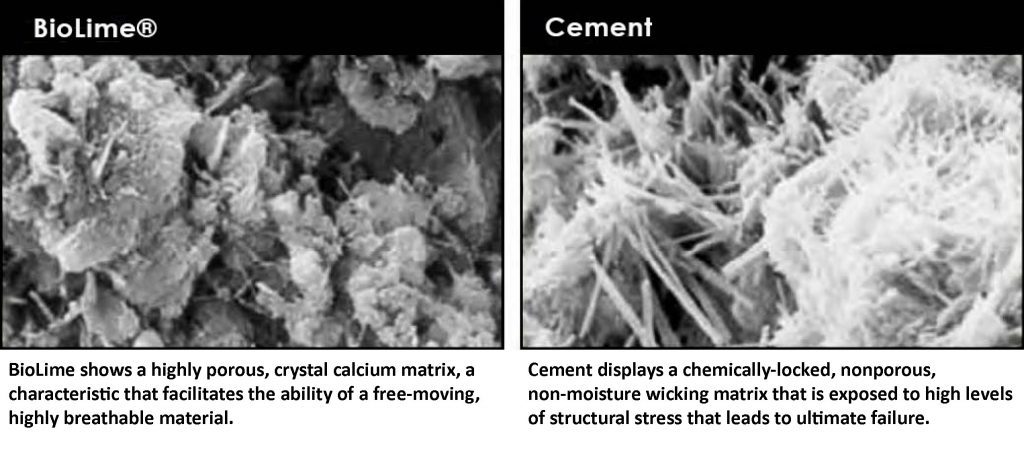
The magnified image of a lime-based building product shows a crystalline, porous structure that allows capillary action to freely release, absorb and reabsorb CO2.
Prior to the invention of Portland cement, building with lime and limestone has sustained architecture through the most challenging environmental conditions throughout history. Unlike cement-based building products, building lime materials absorb CO2 throughout its life cycle. This allows the matrix to slowly cure, resulting in mortars that become highly durable over time. This is a significant benefit over Portland cement-based materials as they do not crack or fracture.
Choosing lime-based building products is a wise decision for achieving truly sustainable architecture. The reassurance of knowing there is a guaranty of offsetting carbon emissions when compared to Portland cement is a sound choice for present and future architecture.
Invest in Lime today for continuing to improve tomorrow.
Sources:
- Lime is a much greener option than cement – https://www.theguardian.com/commentisfree/2007/oct/23/comment.comment
- Carbonation of lime-based materials for direct air capture –
https://www.sciencedirect.com/science/article/pii/S0959652619332007 - British Lime Association: Recarbonation – https://britishlime.org/sustainability/recarbonation.php
- European Lime Association: LCA – https://www.eula.eu/environmental-impact-assessment-lca-life-cycle-assessment/

